C. elegans: a powerful model organism for neurobiology research. C. elegans possesses many advantages as a model animal. These include a simple anatomy of 959 somatic cells, rapid generation time (~3 days), a fully sequenced genome, transparent bodies to facilitate in vivo imaging, and a powerful collection of genetic and molecular tools.
C. elega ns
ns is especially well suited for research in neurobiology given that the axonal wiring and synaptic connectivity of its relatively simple nervous system of 302 neurons is completely described and annotated. While
C. elegans is a simple animal, it shares many of the same biological pathways and genetic mechanisms that are found in more complex organisms.
C. elegans: a model for neural tube
development.
We have shown
that assembly of the ventral nerve cord (VNC) in
C. elegans, an invertebrate, involves some of the same molecular
and cellular mechanisms that drive neural tube formation in vertebrates
(Developmental Cell, 2017). These include rosette-based
convergent extension (CE) and the polarized distribution of VANG-1/Van Gogh, a
core component of the planar cell polarity (PCP) pathway at cell-cell
junctions. We have also identified novel mechanisms, such as the finding that
SAX-3/Robo, better known for roles in cell and axon migration, acts in parallel
with VANG-1/PCP to regulate CE during VNC assembly. Our work suggests that
morphogenesis of the
C. elegans VNC
and the vertebrate neural tube share deep evolutionary
roots. VNC assembly in
C. elegans should
therefore provide an anatomically simple and genetically accessible new model
in which to study neural tube formation. We are currently undertaking large scale
genetic screens followed by whole genome sequencing to identify genes involved
in VNC assembly (easily identifiable as aberrant positioning of motor neurons
in the VNC). The resulting collection of genes should greatly facilitate the
elucidation of cellular and molecular mechanisms involved in nerve cord
development in
C. elegans and thereby
contribute to our understanding of neural tube development in vertebrates.
Figure 1.
Time-lapse imaging (80 minutes) of neuroblast movements during ventral nerve
cord (VNC) assembly.
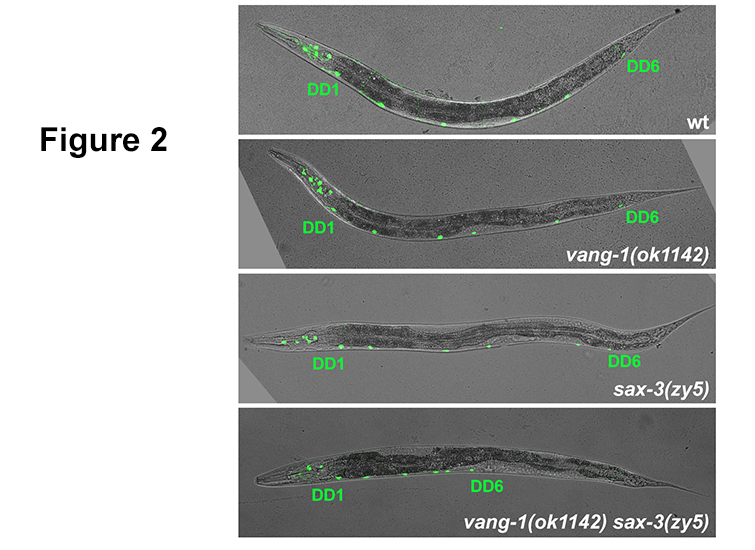
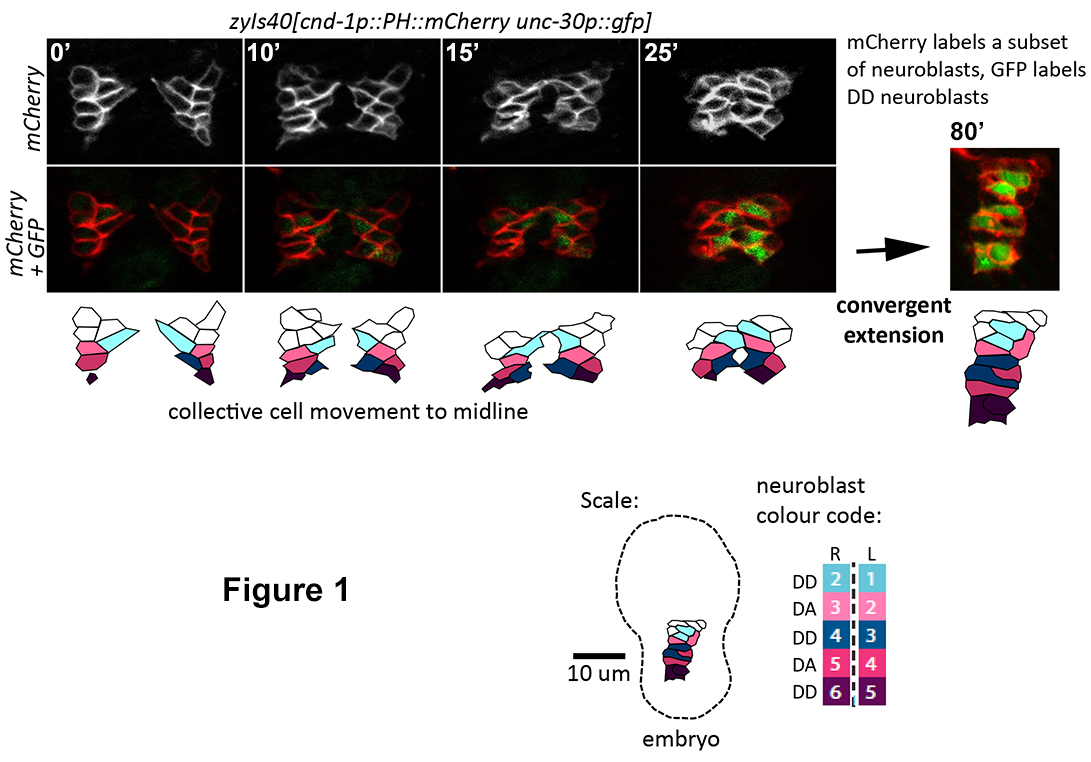 Figure 2. vang-1
Figure 2. vang-1/PCP and
sax-3/Robo mutants display DD neuron position defects resulting from cell intercalation defects during VNC assembly. Severe convergent extension defects in
vang-1 sax-3 double mutants manifest as shorted and anterior VNCs in larval and adult worms.