Gage Lab
Team Leader

Blair Gage
Scientist, Regenerative MedicineWhat We Do
Research Activities
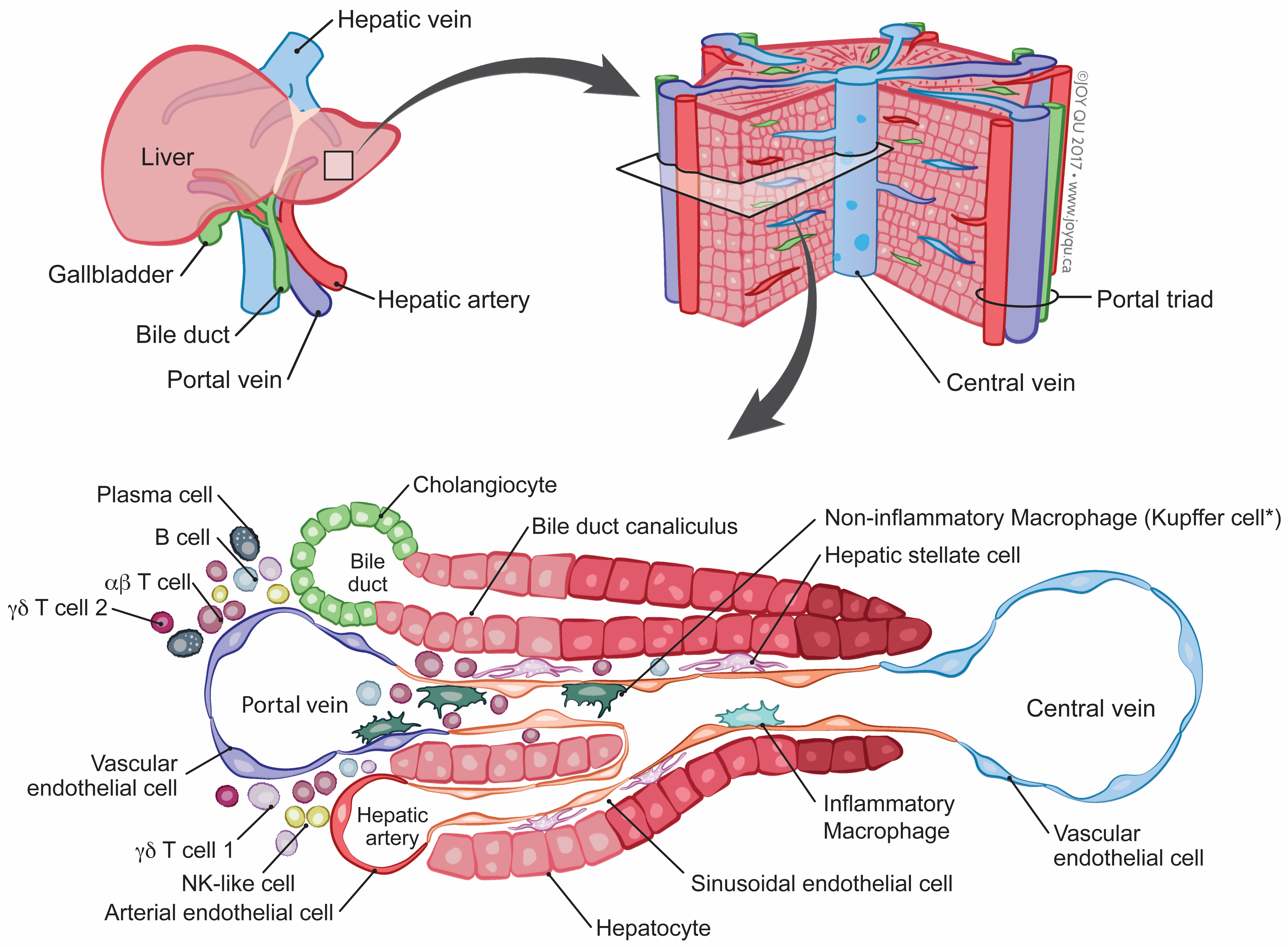
Most of our work focuses on the liver. In Canada, liver disease is on the rise and 1 in 4 Canadians of all ages are affected. Whether acute (eg. drug overdose), chronic (eg. NAFLD, NASH, and cirrhosis), congenital (eg. Hemophilia A), or cancer (eg. liver metastasis), what unifies many liver diseases is that liver endothelium specifically lose normal functions as the disease progresses. In the liver, capillaries known as Liver Sinusoidal Endothelial Cells (LSECs) reside among many other cell types in the liver sinusoid (please see below). In this location, LSECs are functionally specialized for transcellular transport (eg. LDL) biomolecule scavenging (eg. hyaluronan, immunoglobulin, bacterial debris, damaged blood proteins), protein secretion (eg. coagulation factor FVIII) and sustaining liver regeneration via paracrine signalling.
The Liver and the Sinusoid: (MacParland et al. 2018. Article)
The Gage Lab's research centers around three project themes that leverage a common humanized mouse model. Here we use human pluripotent stem cells to model early human development and the formation and specification of endothelial progenitors and venous endothelial cells (VEC) (see below). This work is done in the lab with cell culture technologies that give us access to cells at very early stages of development so we can explore the genetics of early development. With purified VECs generated, we surgically deliver these cells into mouse models where they engraft and grow in the liver of the mice while responding to the liver niche signals of the sinusoid that drive maturation and maintenance of function. Remarkably these human VECs replace over 90% of the mouse liver vasculature leading to near complete humanization of the organs blood vessels. The hPSC-LSECs that reside in the mouse show functions of human LSEC specific fenestration patterns, rapid scavenging, FVIII secretion and extensive transcriptional matching to healthy primary human LSECs.
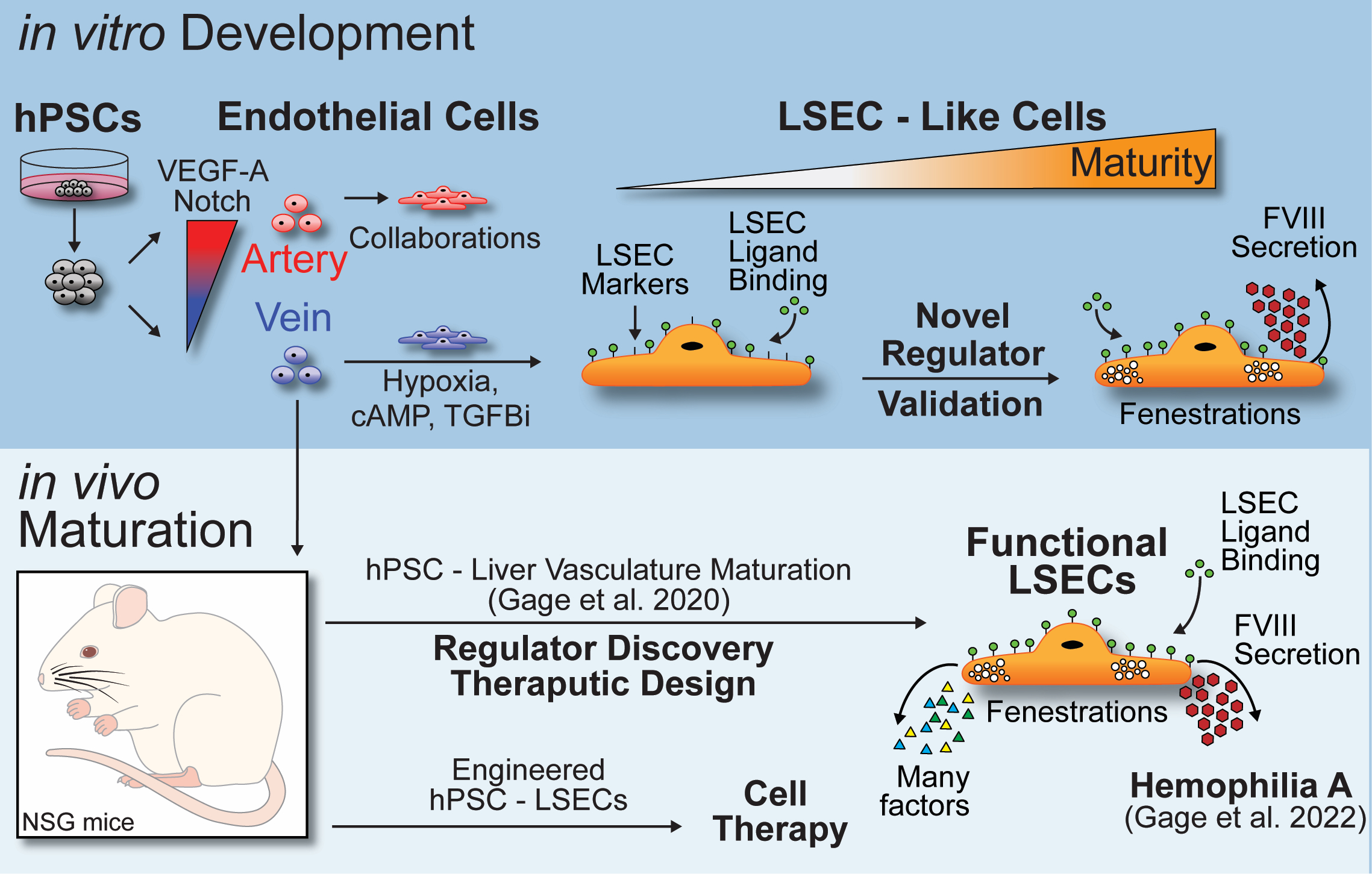
Based on this human developmental and in vivo maturation model, we now focus on three project themes:
1) Regulators of human LSEC functional maturation and maintenance
What signals drive function? What signals sustain these functions lost to disease? We are currently using both candidate and unbiased transcriptomics approaches to identify signalling pathways that control specific LSEC functions. Linking genotype to LSEC phenotype, we use lentiviral vectors, CRISPR-Cas9, and extensive molecular phenotyping (scRNA-seq, flow cytometry, RT-qPCR, histology, and electron microscopy) to reveal direct and indirect functional regulation.
2) Designing endothelial function restorative therapeutics
As we build an understanding of signals that impart and sustain LSEC functions, we reveal therapeutic targets to fight liver disease. For example, acquired liver diseases (drug induced liver injury, NASH, NAFLD, and liver metastasis) all reduce LSEC scavenging and transport functions. We hypothesize that therapeutic rescue of these functions may indirectly rescue other liver functions by supporting other liver cell types (eg. hepatocytes). This work models disease in our humanized mouse model to ensure designed therapeutics provide predicated therapeutics benefits directly or indirectly through human LSECs in vivo.
3) Cell therapy for Hemophilia A and liver disease
Some liver vascular disease are of genetic origin leading to a loss of function in liver endothelium. An example of this is Hemophilia A where all the body’s cells fail to produce sufficient FVIII protein to regulate blood clotting. Since LSECs are numerous and naturally make high levels of FVIII, we previously applied our hPSC-based VEC cell therapy model to Hemophilia A mice collaboratively with Dr. Antonia Follenzi (Università, del Piemonte Orientale, Italy). Mice receiving hPSC-VEC cell therapy corrected their severe bleeding to a mild form due to the bioactive hPSC-LSEC produced FVIII. We are currently working to advance this approach to become free from immune rejection constraints and leverage the other natural and engineered proteins that hPSC-LSECs make to fight liver disease from within the organ.
Selected Publications
1. Gage BK, Liu JC, Innes BT, MacParland SA, McGilvray ID, Bader GD, Keller GM. Generation of Functional Liver Sinusoidal Endothelial Cells from Human Pluripotent Stem Cell-Derived Venous Angioblasts. Cell Stem Cell. (2020) 27(2): 254-269 e259. Article.
- This study established the core mouse model used in the Gage lab where mouse liver vasculature (LSECs) are replaced by functionally mature hPSC-derived LSECs.
- Exploration of published scRNAseq data of hPSC-LSECs can be found collaboratively here with Dr. Gary Bader (U of Toronto).
2. Gage BK, Merlin S, Olgasi C, Follenzi A, Keller GM. Therapeutic Correction of Hemophilia A by Transplantation of hPSC-derived Liver Sinusoidal Endothelial Cell Progenitors. Cell Rep. (2022) 39(1): 110621. Article.
- This study developed a new higher yield differentiation protocol to make hPSC-derived venous endothelial cells with enhanced liver engraftment potential.
- In collaboration with Dr. Antonia Follenzi (Università, del Piemonte Orientale, Italy), this study also revealed that hPSC-VEC cell therapy can functionally correct severe bleeding phenotypes seen in Hemophilia A mice paving the way for future cell therapy development.
3. Kent GM, Atkins MH, Lung B, Nikitina A, Fernandes IM, Kwan JJ, Andrews TS, MacParland SA, Keller GM, Gage BK. Human liver sinusoidal endothelial cells support the development of functional human pluripotent stem cell-derived Kupffer cells. Cell Rep. (2024) 43 (8): 114629. Article.
- This study used our NSG-LSEC humanized mice as an in vivo source of human M-CSF to facilitate stable human macrophage engraftment in the liver.
- hPSC-derived yolk sac (primitive and MPP/EMP programs) macrophage progenitors stably engraft NSG-LSEC mice and functionally mature to Kupffer cells with homogenous transcriptional signature (MARCO, TIMD4, FOLR2, IL10 etc.) and robust phagocytotic and erythrophagocytotic capacity.
- Cord blood HSPC progenitors fail to show a MARCO signature but expanded Cord blood-derived macrophage progenitors can generate MARCO Kupffer cells.
- Check out the scRNAseq data including the last mouse liver cell that the human Kupffer cells ate as an ongoing collaboration with the Andrews lab (UWO) here: Data Portal.