Dr. Wang's research program focuses on dissecting the molecular mechanisms that regulate neural stem cell reprogramming and differentiation in the context of neurological diseases including Alzheimer's Disease, Stroke and Multiple Sclerosis, and further using this understanding to rationally design and test therapies for neural injury and degeneration. Several ongoing research projects are described below.
1.
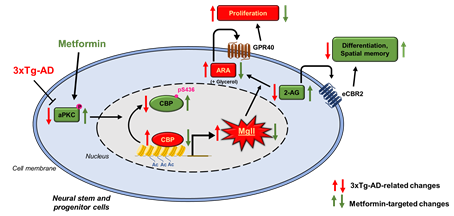
Molecular control of lipid metabolism in brain aging: We have identified the role of atypical protein kinase C (aPKC) mediated Ser 436 phosphorylation in CBP, a histone acetyltransferase (HAT), as an important mechanism that sustains hippocampal neuronal differentiation, maturation, and memory during normal aging (
Stem Cell Reports, 2016). We also demonstrate that metformin, an FDA-approved anti-diabetic drug, can activate the aPKC-CBP pathway to promote NPC neuronal differentiation and improve hippocampus-associated memory in vivo (
Stem Cell Reports, 2015, Cell Stem Cell, 2012). Our recent publication (
Theronostics, 2020) reveals that when the aPKC-CBP pathway is impaired in an AD animal model during pathological aging, monoacylglycerol lipase (Mgll) levels are highly upregulated in hippocampi to reduce neuronal differentiation and impair spatial memory in the AD animal model. Intriguingly, these AD-associated deficits can be rescued by metformin treatment through reactivating the aPKC-CBP mediated Mgll gene repression. Ongoing research program continue to identify extrinsic source of Mgll and its roles in regulating neural stem cell behavior in vivo and aim to translate the findings into clinical applications.
2.
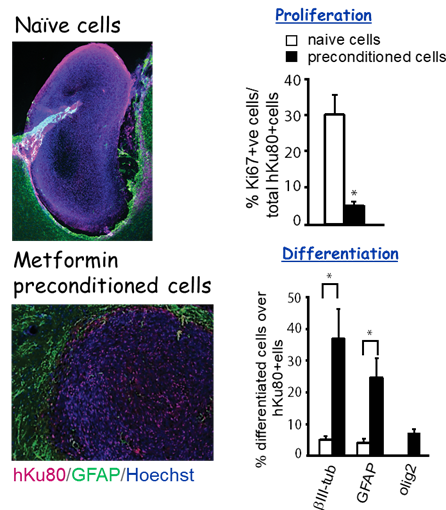
Optimizing cell-based therapies to promote neurovascular regeneration and behavioural recovery after ischemic stroke. We recently used a rat Endothelin-1 (ET-1) focal ischemic stroke model to demonstrate that preconditioning of human iPSC derived neural stem cells (hiPSC-NSCs) with metformin before transplantation into the stroke-damaged brains improves engraftment and regeneration capabilities of hiPSC-NSCs and ultimately accelerates gross motor recovery and reduces infarct volume (
Stem cells and Development, 2018). Ongoing research project continues to optimize cell-based therapies to promote neurovascular regeneration and behavioural recovery following ischemic stroke.
3.
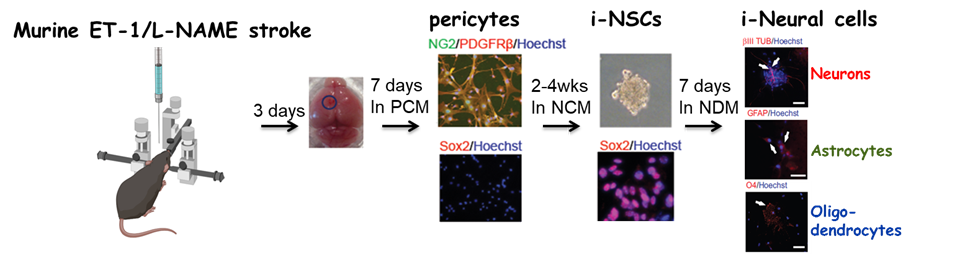
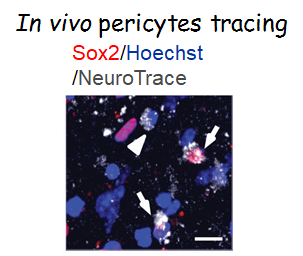
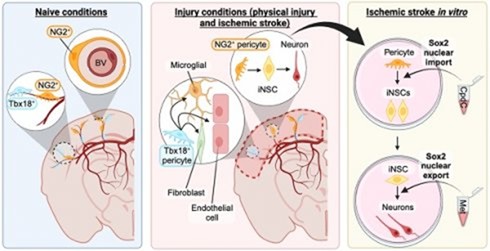
Targeting in vivo cellular reprogramming for post-stroke neural regeneration and recovery. We used a mouse Endothelin-1 (ET-1) focal ischemic stroke model to identify a transient population of locally-derived NSCs (i-NSCs) that are reprogrammed
from pericytes in the injury site shortly after stroke. We further show that the permanent deletion of the aPKC-CBP pathway
enhances the pericyte reprogramming efficiency to multipotent NSCs, consequently
exhausting proliferative pericytes and compromising the vascular remodeling to
impair post-stroke functional recovery (
Stem Cell Reports, 2017). In our recent published paper (
Theranostics, 2024), we revealed that two distinct subtypes of pericytes possess different reprogramming potencies in response to physical and ischemic injuries using single cell RNA-seq combined with immunohistochemistry. We also developed a genomic integration-free methodology to reprogram human pericytes into functional neurons by targeting NG2+ pericytes in the dish. Current ongoing research project is to define ways to not only enhance neural reprogramming of ischemia-activated pericytes into i-neurons, but also reduce post-stroke hostile microenvironment for maximizing post-stroke local neurogenesis.