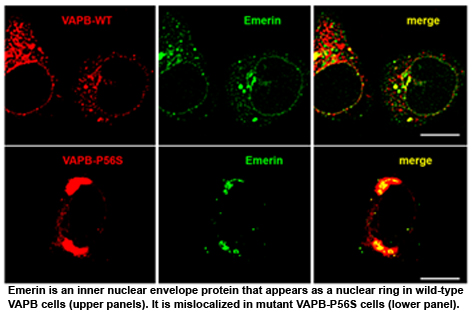
John Ngsee
Ph.D.
Affiliate Investigator, Neuroscience
Ottawa Hospital Research Institute
Associate Professor, Cellular and Molecular Medicine
University of Ottawa
Contact
613-562-5800 x2251
Cellular and Molecular Medicine University of Ottawa 451 Smyth Road, RGN 2442 Ottawa, ON, Canada, K1H 8M5
Bio
Education:
1972-76 B.Sc. (Honours) in Physiology, University of British Columbia
1976-78 M.Sc. in Physiology, University of British Columbia, Dr. Nadine Wilson
1981-86 Ph.D. in Biochemistry, University of British Columbia, Dr. Michael Smith
Research Experience:
1986 Postdoctoral fellow, Pathology, University of British Columbia, Dr. Shirley Gillam
1987-93 Postdoctoral fellow, Molecular and Cellular Physiology, Stanford University, Dr. Richard H. Scheller
1993-present Senior Scientist/ Associate Professor, Ottawa Hospital Research Institute, University of Ottawa