Flow Cytometry and Cell Sorting Facility
Explore Flow Cytometry and Cell Sorting Facility
Instruments and software
Our Flow Cytometry and Cell Sorting's equipment includes a BeckmanCoulter MoFlo XDP, a SONY MA900, a BD LSR Fortessa, and a Cytek Aurora. The sections below provide more detailed information on these instruments.
You will also see a list of the software available for the analysis of flow cytometry data. Please contact the instrument administrators for more details.
Instruments
The Ottawa Hospital Sprott Centre for Stem Cell research
5th floor Critical Care Wing, Room 5145
501 Smyth Road
Ottawa, Ontario K1H 8L6
The BeckmanCoulter MoFlo XDP is a state-of-the-art instrument, equipped with five excitation laser lines (UV, 405nm, 488nm, 561nm, and 640nm), which can be used for multi-parameter experiments and applications requiring high speed sorting and/or sorting of four sub-populations simultaneously. A designated operator processes the samples.
The MoFlo XDP is a true 32-bit highresolution digital system, which enables accurate sorting rates of up to 70,000 events per second and analysis rates of up to 100,000 events per second. The precision of the electronics platform combined with the reliably stable MoFlo fluidics allows accurate sorting into as much as 1536-well plates.
Configuration
Jet-in-air system
Five excitation lasers:
Coherent Genesis CX 355nm (UV)
Coherent Cube 405nm
Coherent Saphire 488nm
Coherent Saphire 561nm
Coherent Cube 640nm
Two light scatter channels and 15 fluorescence detectors:
2 fluorescence detectors on the 355nm laser path
3 fluorescence detectors on the 405nm laser path
5 fluorescence detectors on the 488nm laser path
2 fluorescence detectors on the 561nm laser path
3 fluorescence detectors on the 640nm laser path
A policy has been implement which states that users of the facilily recognize the potential for contamination of their samples and that use of the MoFlo facility includes this assumed risk. The MoFlo does not generally have a high incident rate with contamination issues; however, as with any sample manipulation, the posbility does exist. In the case of post-sorting contamination, it is heavily recommended that the users notify the facility so that proper action can be taken.
The Ottawa Hospital Cancer Centre
3rd floor, South wing, room 3340E
501 Smyth Road
Ottawa, Ontario K1H 8L6
The SONY MA900 is a user-operated cell sorting instrument capable of detecting up to 12 different fluorescent markers, plus forward and side scatter parameters, and the ability to sort into different size tubes and plates.
Optics specifications
The SONY MA900 is equipped with 4 excitation lasers (405 nm, 488 nm, 561 nm, 638 nm) in a dual axis optical system, (colinear system between 488 / 561 nm lasers and 405 / 638 nm lasers.
The instrument is capable of detecting up to 12 different fluorescent parameters (5 parameters in 488 / 561 nm lasers and 7 parameters in 405 / 638 nm lasers) plus forward and side scatter parameters.
Fluidics and sorting specifications
The instrument uses an auto loading chamber where you can load your samples in different tube sizes, ranging from 0.5 mL to 15 mL. The loading chamber is also temperature controlled and has an agitation unit to maintain the cells in suspension during the sort.
The sorting chamber also allows for different sizes of tubes, from 0,5 mL to 50 mL, and it is capable of doing from 2-way to 4-way sorts. The sorting chamber has outstanding set up for different size plates, from 6- to 384-well plates.
The main feature of the SONY MA900 instrument is the microfluidics chip-based technology, which helps users to easily install and set up the chip/nozzle system into the instrument and automatically calibrate it. The facility has 3 different size nozzle chips which can be used depending on the type of cells the users will be sorting:
70 um nozzle
100 um nozzle
130 um nozzle
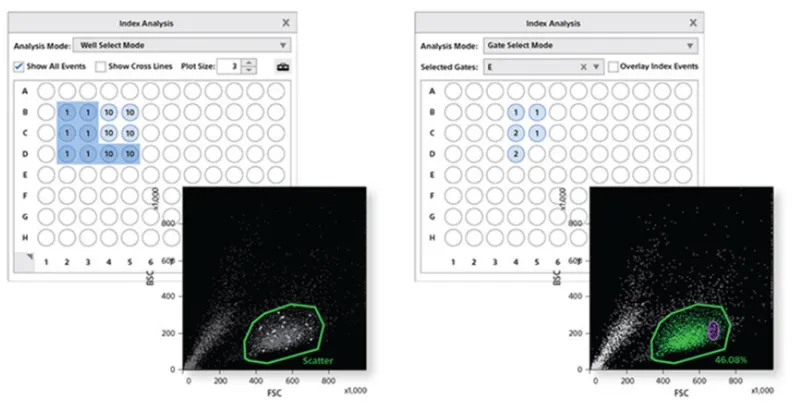
Index sorting feature
Index sorting software records the X and Y coordinates of each event sorted into a multi-well device. This very precise and easy to use software brings powerful capabilities to research, enabling you to track the scatter and fluorescence intensity of individual cells sorted in each well.
These index sort options allow you to perform meta-analysis of data for several applications. For example, clonal variability can be studied based on the expression levels of the fluorescent protein or surface markers. Also, researchers can integrate phenotypic data with mRNA expression analysis of the sorted cells.
The Ottawa Hospital Sprott Centre for Stem Cell Research
Critical Care Wing, 5th Floor, Room W5215
501 Smyth Road
Ottawa, Ontario K1H 8L6
The BD LSR Fortessa cell analyzer offers the ultimate in choice for flow cytometry, providing power, performance, and consistency. Designed to be affordable and expandable, the BD LSRFortessa has the flexibility to support the expanding needs of multicolor flow cytometry assays.
Optics specifications
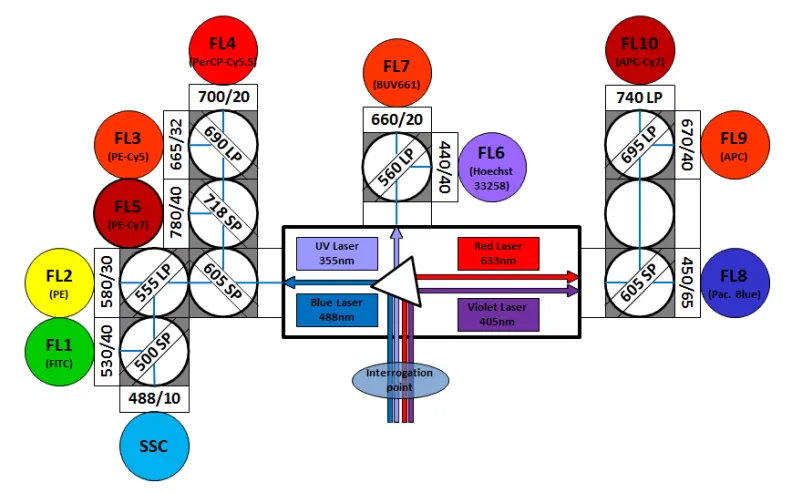
The BD LSR Fortessa is equipped with 5 lasers (355nm, 405nm, 488nm, 561nm, 633nm). Arrange in octagon- and trigon-shaped optical pathways, their novel design efficiently maximizes signal detection and increases sensitivity and resolution. This allows researchers to identify cells, especially dim and rare cell populations, optimizing multicolor assays and panel design for superior results.
This configuration allows us to detect up to 18 different fluorescent parameters: 3 parameters from 355nm laser, 5 parameters from 405nm laser, 2 parameters from 488nm laser, 5 parameters from 561nm laser and 3 parameters from 633nm laser.
| LASER | FILTER | FLUOROCHROME |
|---|---|---|
| UV (355nm) | 379/28 | BUV395 |
| 670/25 | BUV661 | |
| 740/35 | BUV737 | |
| Violet (405nm) | 450/50 | BV421, V450, Pacific Blue |
| 525/50 | BV510, V500, Am Cyan | |
| 660/20 | BV650 | |
| 710/50 | BV711 | |
| 780/60 | BV786 | |
| Blue (488nm) | 530/30 | FITC, GFP, YFP, Alexa Fluor 488, BB 515, Venus |
| 710/50 | PerCP-Cy5.5 | |
| Yellow-Green (561nm) | 586/15 | PE, Ds Red, TdTomato, Alexa Fluor 546 |
| 610/20 | PE-Texas Red, m Cherry, PE-Alexa 610, PE-CF594, PI | |
| 670/30 | PE-Cy5, 7-AAD, PE-Alexa 647 | |
| 710/50 | PE-Cy5.5 | |
| 780/60 | PE-Cy7, PE-Alexa 750 | |
| Red (633nm) | 670/14 | APC, Alexa Fluor 647 |
| 730/45 | Alexa Fluor 700 | |
| 780/60 | APC-Cy7, Zombie NIR |
Fluidics
The fluidics system is pressure driven. Hydrodynamic focusing forces sample cells through the cuvette flow cell, where they are interrogated. The flow cell is in fixed alignment with the laser and gel-coupled to the collection optics. This design ensures that the laser is precisely focused on the sample stream and the maximum amount of emitted light can be collected for added sensitivity in multicolor applications. Fixed alignment also minimizes startup time, improves experiment-to-experiment reproducibility, and enables automated daily quality control.
Fluidic sensors maintain constant pressure, while a fluidics monitoring system warns when sheath fluid is low, empty, or when the waste container is full.
BDTM High Throughput Sampler option
To improve experimental workflow, the optional BDTM High Throughput Sampler (HTS) provides rapid, fully automated sample acquisition from 96- and 384-well microtiter plates. In high-throughput mode, the HTS option can speed through a 96-well plate in fewer than 15 minutes with less than 0.5% sample carryover from one well to the next. Low carryover is essential in research applications to ensure sample purity and data integrity.
Fast acquisition speed is achieved by synchronizing two high-precision pumps for sample mixing, sample injection, and probe washing. Standard throughput mode can be selected for acquisition of larger sample volumes.
The Ottawa Hospital Cancer Centre
3rd floor, South Wing, Room 3325E
501 Smyth Road
Ottawa, Ontario K1H 8L6
A prodigy incorporating a unique combination of innovative technologies that takes flow cytometry to the next level of performance and flexibility.
With up to five lasers, three scattering channels, and 64 fluorescence channels, the Aurora system suits every laboratory’s needs, from simple to high-complexity applications. A paradigm shifting optical design provides unprecedented flexibility, enabling the use of a wide array of new fluorochrome combinations without reconfiguring your system for each application. The state-of-the-art optics and low-noise electronics provide excellent sensitivity and resolution.
Optics specifications
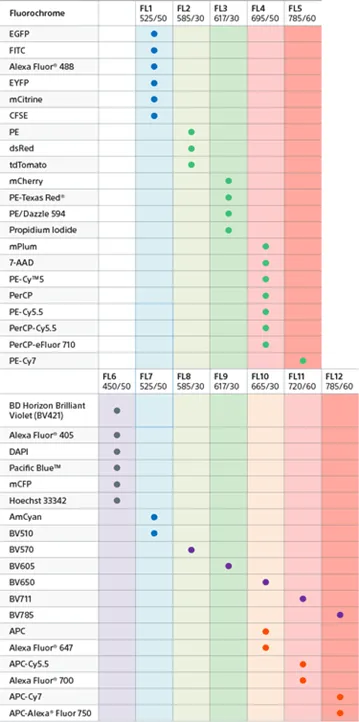
The Cytek Aurora is equipped with 5 lasers (355nm, 405nm, 488nm, 561nm, 640nm), allowing the researchers to detect up to 40 different demonstrated fluorophores. Due to its large detection capability, there is no need to change the configuration of the optical filters.
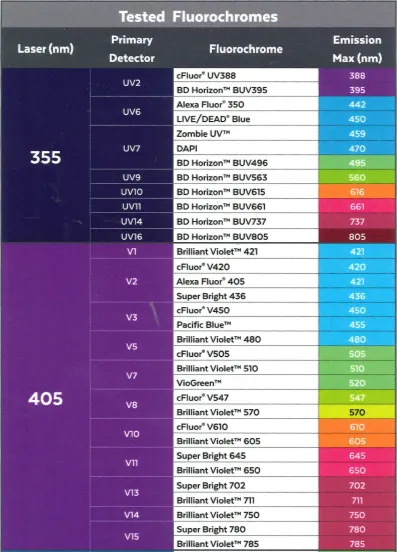
Deep immunoprofiling
The Cytek Aurora’s use of full spectrum flow cytometry combined with the SpectroFlo® software’s real-time unmixing capability provides greater fluorochrome choice and panel flexibility and allows users to quickly visualize data and statistics. All this results in the ultimate flow cytometry solution for deep immunoprofiling, from 24 colors all the way to 40 colors.
Automated Sample Loader
The ASL offers more versatility when running your samples at high-throughput. In addition to acquisition from 96-well plates, the ASL is compatible with 96-deep well plates and 40-tube racks. For each carrier type, Cytek has provided preset mixing speeds and frequencies, which are also fully customizable to meet your individual experimental requirements. The ASL is designed to streamline experimental workflows and integrates seamlessly into the Aurora system
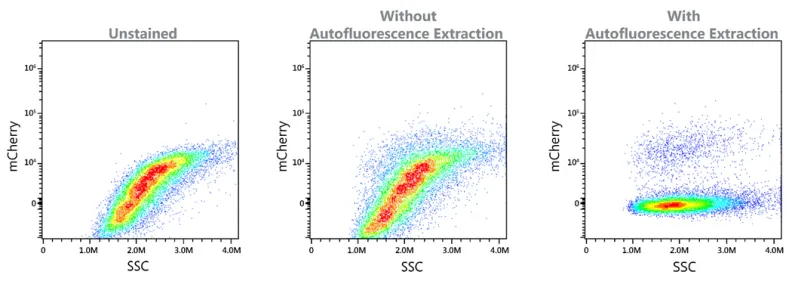
Autofluorescence extraction
The Aurora system’s implementation of full spectrum flow cytometry enables the use of autofluorescence extraction to further improve data clarity. Certain sample types, such as yeast and tumor samples, present the challenge of high autofluorescence. For these challenging applications involving highly autofluorescent particles, let the software's autofluorescence extraction tool bring new levels of resolution.
Data analysis software
- Available for MoFlo users, please contact instrument operator.
- Data acquisition on MoFlo.
- General data analysis.
- Database management.
- Histogram substraction and overlays.
- Up to 50 million event files.
- Available for SONY MA900 users, please contact instrument operator.
- Data acquisition on SONY MA900.
- General data analysis.
- Available for BD LSR Fortessa users.
- Data acquisition on BD LSR Fortessa.
- General data analysis.
- Available for MoFlo users, please contact instrument operator.
- General data analysis.
- Database management.
- Histogram substraction and overlays.
- Up to 50 million event files.
- Available for Cytek Aurora users.
- Data acquisition on Cytek Aurora.
- Spectral & Flow Cytometry data analysis.
1 computer at the Ottawa Hospital Cancer Centre contains the software for the usage of researchers.
- General data analysis.
- Database management.
- Histogram substraction and overlays.
- Up to 50 million event files.
2 computers at the Ottawa Hospital Sprott Centre for stem cell research contain the software for the usage of the researchers.