Pierre Mattar
PhD
Senior Scientist, Regenerative Medicine
Ottawa Hospital Research Institute
Assistant Professor, Cellular and Molecular Medicine
University of Ottawa
Clifford, Gladys and Lorna J. Wood Chair for Research in Vision
Research Goals and Interests
News
Publications
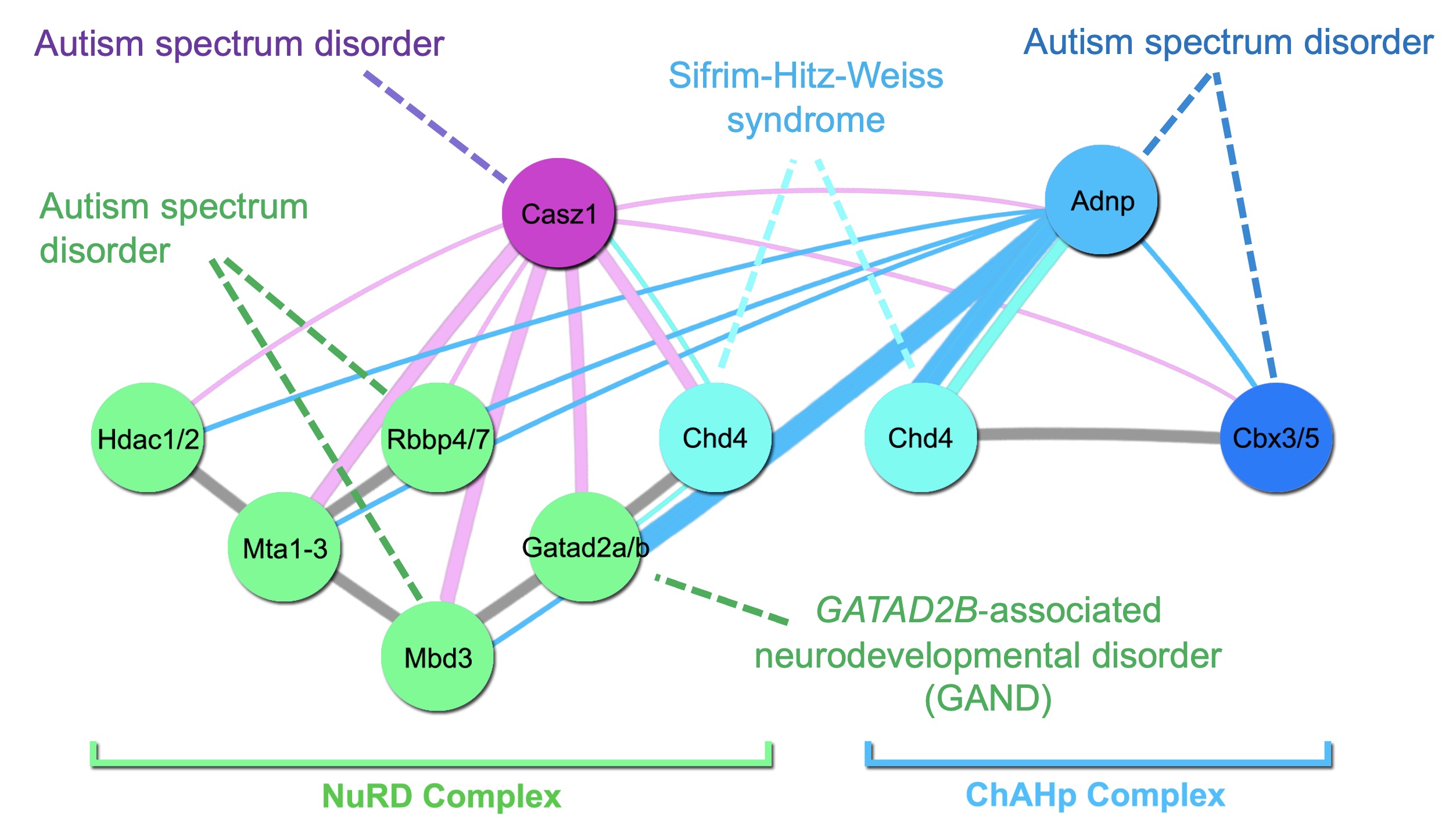
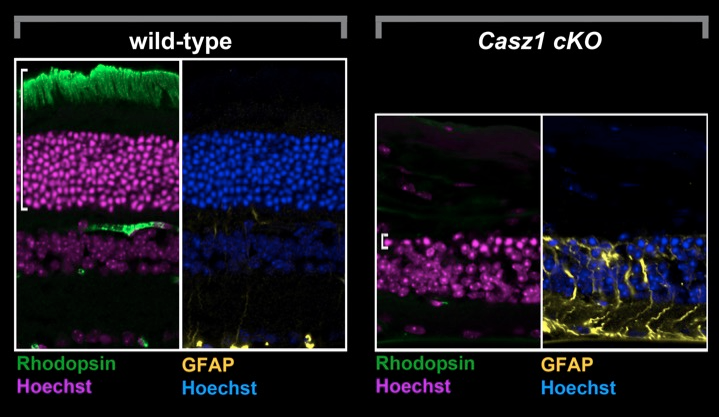
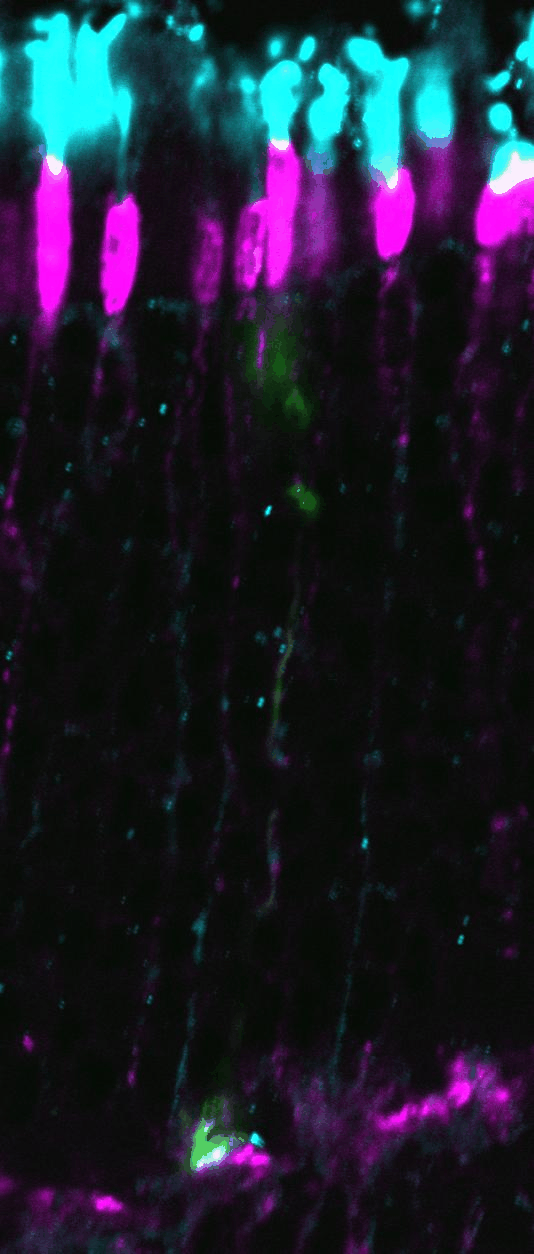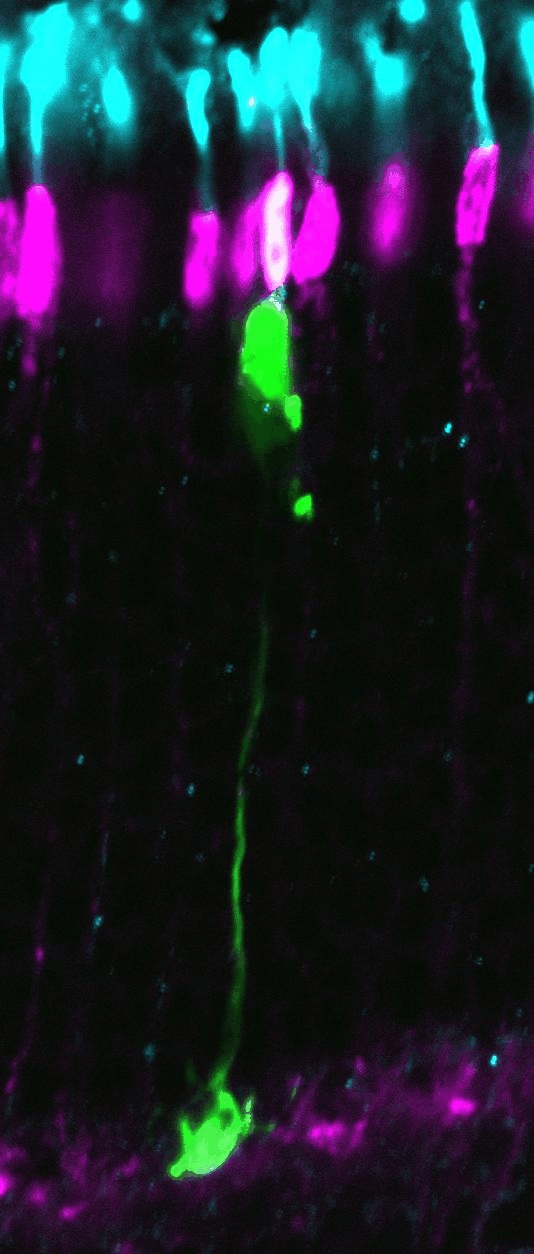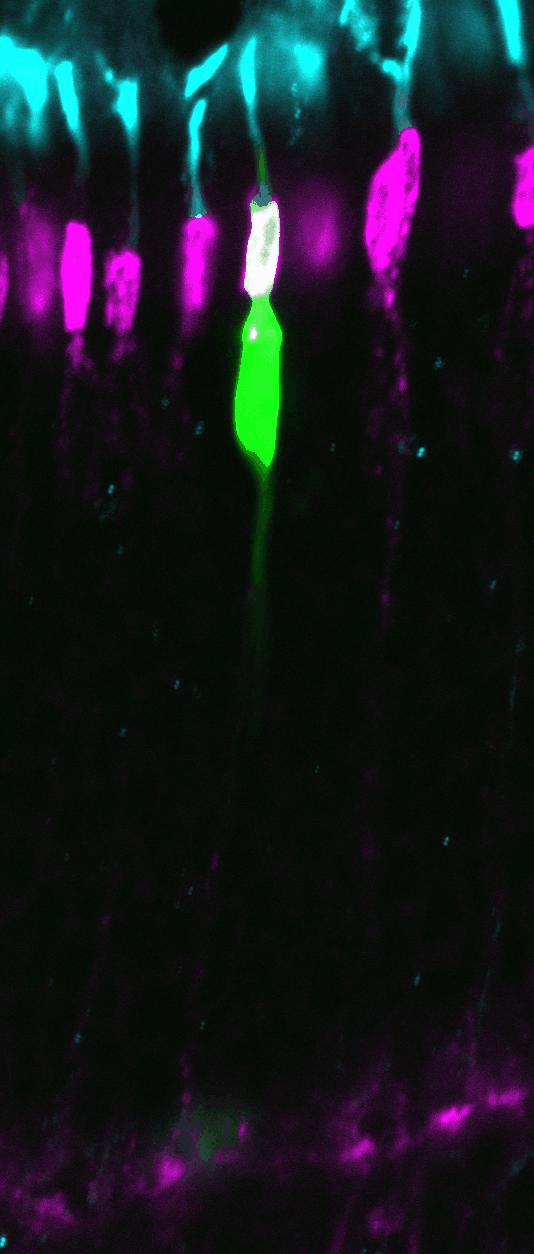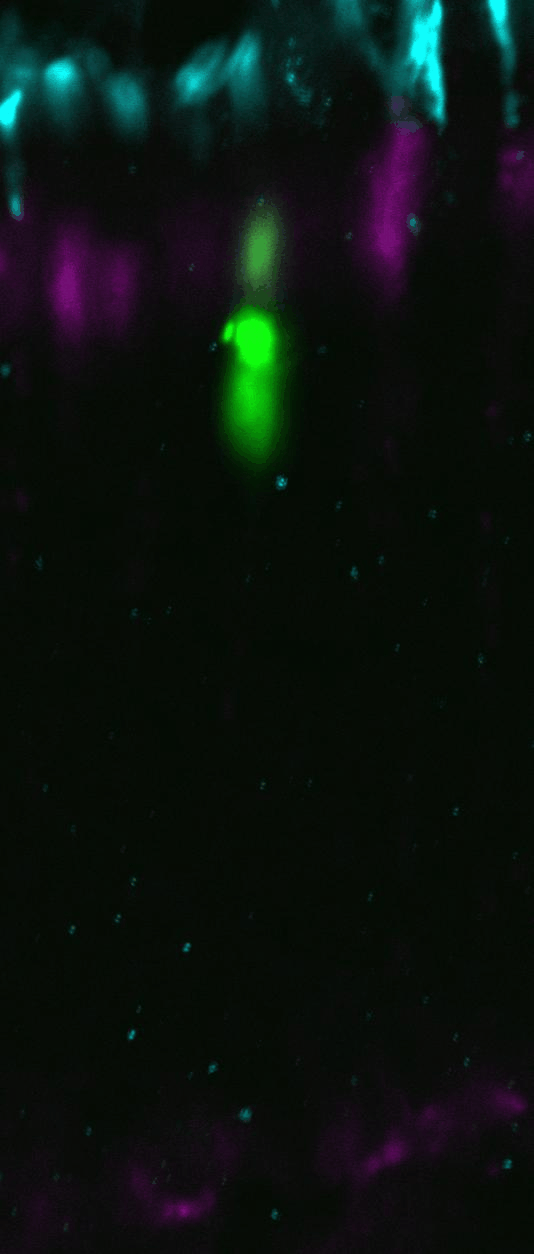
Chd4 remodels chromatin to control retinal cell type specification and lineage termination
2025-10-15 Go to publicationThe chromatin remodeler ADNP regulates neurodevelopmental disorder risk genes and neocortical neurogenesis.
2025-01-14 Go to publicationChd4 remodels chromatin to control retinal cell type specification and lineage termination
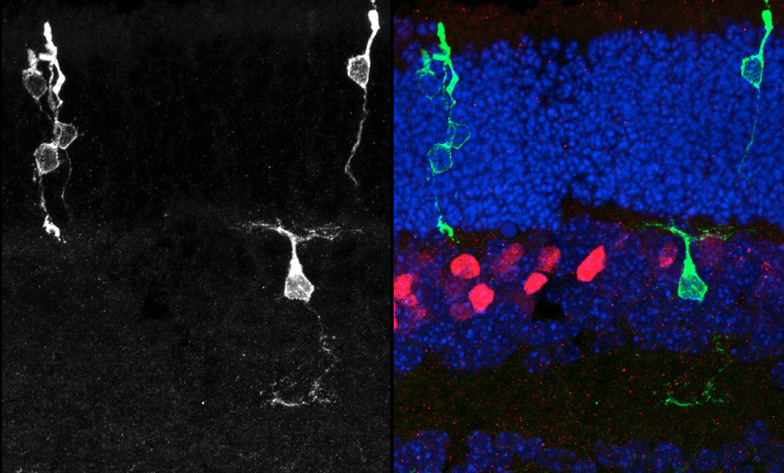
2025-01-10 Go to publicationPten regulates endocytic trafficking of cell adhesion and Wnt signaling molecules to pattern the retina.
2024-03-27 Go to publicationLamin A upregulation reorganizes the genome during rod photoreceptor degeneration
2023-10-25 Go to publicationRelated Research at The Ottawa Hospital
- Regenerative Medicine Program
- Glaucoma
- Intellectual disabilities
- Macular degeneration
- Retinal disease
- Vision
- Autism
- Autoimmune diseases of the nervous system
- Discovery research
- Genetics
- Genomics
- Bioinformatics
- Model organisms
- Molecular and cellular biology
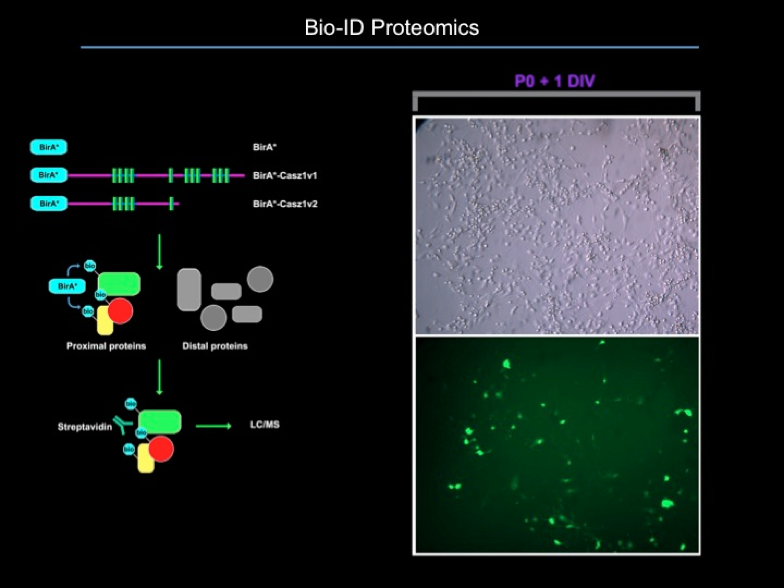
- Proteomics
- Regenerative medicine
- Epigenetics
- Transgenic/knockout models
- Cell therapy
- Gene expression
- Systems biology
- Stem cells